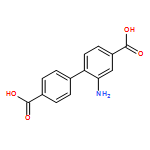
2-氨基-4,4'-联苯二甲酸
2-Aminobiphenyl-4,4'-dicarboxylic acid
CAS:1240557-01-0
分子式:C14H11NO4
分子量:257.24144
请选择
品牌 / 纯度 / 包装
产品名称:2-氨基-4,4'-联苯二甲酸
其他名称:H2BPDC-NH2; H2amineBPDC,2-amino-[1,1'-biphenyl]-4,4'-dicarboxylic acid
CAS: 1240557-01-0
其他名称:H2BPDC-NH2; H2amineBPDC,2-amino-[1,1'-biphenyl]-4,4'-dicarboxylic acid
CAS: 1240557-01-0
结构信息
| 分子式 | C14H11NO4 | 分子量 | 257.24144 | ||
| 性状照片 |  |
||||
性质说明
1) 1240557-01-0溶于DMF、DMSO等
2) 2-8℃ 密封保存
2) 2-8℃ 密封保存
合成应用
1) 可作为配体用于合成MOF材料: UiO-67-m-NH2 [r4, r5]; DUT-5 amine [r2]; N3/NH2-bMOF-100 [r3]
表征图谱
参考文献
1) R. K. Deshpande, J. L. Minnaar, S. G. Telfer, Angew. Chem. Int. Ed. 2010, 49, 4598– 4602, DOI:10.1002/anie.200905960 ; Thermolabile Groups in Metal–Organic Frameworks: Suppression of Network Interpenetration, Post‐Synthetic Cavity Expansion, and Protection of Reactive Functional Groups
2) M. Gotthardt, S. Grosjean, T. Brunner, J. Kotzel, A. Gänzler, S. Wolf, S. Bräse, W. Kleist, Dalton Trans., 2015,44, 16802-16809 DOI: 10.1039/C5DT02276B ; Synthesis and post-synthetic modification of amine-, alkyne-, azide- and nitro-functionalized metal–organic frameworks based on DUT-5
3) C. Liu, T. Luo, E. Feura, C. Zhang, N. Rosi, J. Am. Chem. Soc. 2015, 137(33), 10508-10511, DOI:10.1021/jacs.5b06780 ; Orthogonal Ternary Functionalization of a Mesoporous Metal–Organic Framework via Sequential Postsynthetic Ligand Exchange
4) Katz, M.; Brown, Z.; Colon, Y.; Siu, P.; Scheidt, K.; Snurr, R.; Hupp, J.; Farha, O.; Chem. Commun., 2013,49, 9449-9451, DOI: 10.1039/C3CC46105J ; A facile synthesis of UiO-66, UiO-67 and their derivatives
5) Peterson, G.W.; Moon, S.; Wagner, G.; Hall, M.; De Coste, J.; Hupp, J.; Farha, O.; Inorg. Chem. 2015, 54, 20, 9684-9686, DOI: 10.1021/acs.inorgchem.5b01867 ; Tailoring the Pore Size and Functionality of UiO-Type Metal–Organic Frameworks for Optimal Nerve Agent Destruction